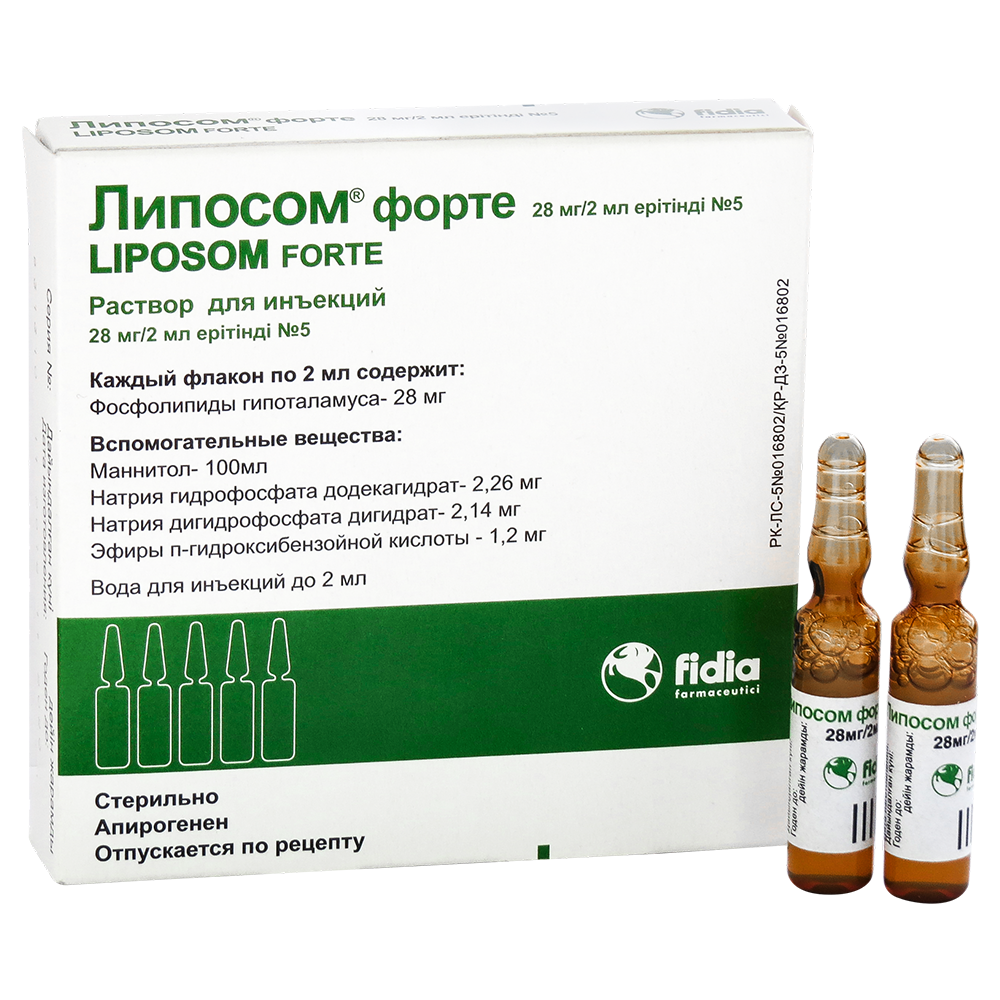
LIPOSOM FORTE
Field of Activity:
Application:
Release form:
Composition:
Liposome® forte International nonproprietary name No Dosage form Solution for injection, 28mg/2ml Compound 2 ml of solution contain active substance – hypothalamic phospholipids 28.0 mg, excipients: mannitol, sodium hydrogen phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate, esters p-hydroxybenzoic acid, water for injection. Description White to cream-colored opalescent solution with a characteristic odor. Pharmacotherapeutic group Drugs […]
Liposome® forte
International nonproprietary name
No
Dosage form
Solution for injection, 28mg/2ml
Compound
2 ml of solution contain
active substance - hypothalamic phospholipids 28.0 mg,
excipients: mannitol, sodium hydrogen phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate, esters
p-hydroxybenzoic acid, water for injection.
Description
White to cream-colored opalescent solution with a characteristic odor.
Pharmacotherapeutic group
Drugs for the treatment of diseases of the nervous system.
Other psychostimulants and nootropics.
ATX code N06ВХ
Pharmacological properties
Pharmacokinetics
After administration of the drug parenterally, metabolic processes were studied both in terms of assessing total radioactivity
and at the cellular level. Research data shows that the molecules are stable in the blood and reach brain cells.
Pharmacodynamics
Parenteral administration of hypothalamic phospholipids can activate hypothalamic metabolism
by increasing the turnover of dopamine, tyrosine hydroxylase and adenylate cyclase with subsequent accumulation
of cyclic AMP. This pharmacological effect is reflected especially on the functions of the hypothalamic-pituitary system.
By influencing the physicochemical properties of neuronal membranes, hypothalamic phospholipids alter
the adaptation of central neuron receptors to treatment.
Indications for use
An adjuvant in the treatment of metabolic cerebral disorders due to neuroendocrine disorders.
Directions for use and doses
Liposome® forte is administered 2 ml intramuscularly or intravenously
1 per day. The course of treatment is determined by the doctor individually.
Side effects
No adverse side effects were detected.
If side effects occur that are not listed in the instructions, you should consult a doctor.
- allergic reactions (skin itching, urticaria, rash)
Contraindications
- established hypersensitivity to the components of the drug
- presence of infection or damage to the skin in the area
injections
Drug interactions
Clinically significant interactions of the drug with other drugs have not been established.
Liposome® forte can be administered simultaneously with other pharmaceutical products and,
in particular, with antipsychotics, with drugs against hyperprolactinemia, with tricyclic
antidepressants (reduces the delay of action and increases efficiency) and with cardiac drugs.
Special instructions
The drug does not entail any special warnings or precautions for use.
Do not use the drug with damaged or opened packaging.
Use in pediatrics
The drug is not recommended for use in children due to the lack of data on the safety and effectiveness of use in pediatrics.
Pregnancy and lactation
During pregnancy and lactation (breastfeeding), the drug should be used under strict medical supervision.
Features of the effect of the drug on the ability to drive vehicles and potentially dangerous mechanisms
Liposome® forte does not affect a person’s ability to drive vehicles or engage in other
potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
No overdose symptoms were identified when treated with recommended doses.
In case of accidental overdose, treatment is symptomatic.
Release form and packaging
Injection solution for intramuscular or intravenous use.
2 ml in orange glass ampoules type I.
5 ampoules each in a blister pack made of polyvinyl chloride film.
1 blister pack together with instructions for medical use in the state and Russian languages are placed in a cardboard box.
Storage conditions
Store at a temperature not exceeding 25°C.
Keep out of the reach of children!
Conditions for dispensing from pharmacies
On prescription
Manufacturer/Packager
Fidia Pharmaceutisi S.P.A.
35031, Abano Terme, Via Ponte della
Fabbrica, Z/A, Italy
Registration Certificate Holder
Fidia Pharmaceutisi S.P.A.
35031, Abano Terme, Via Ponte della
Fabbrica, Z/A, Italy

[contact-form-7 id="327" title="Contact Us"]
